Guna Collagen Prescribing Information
Package insert / product label
Generic name: sus scrofa collagen
Dosage form: injection, solution
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Drug Abuse and Dependence
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- References
- Storage and Handling
- Patient Counseling Information
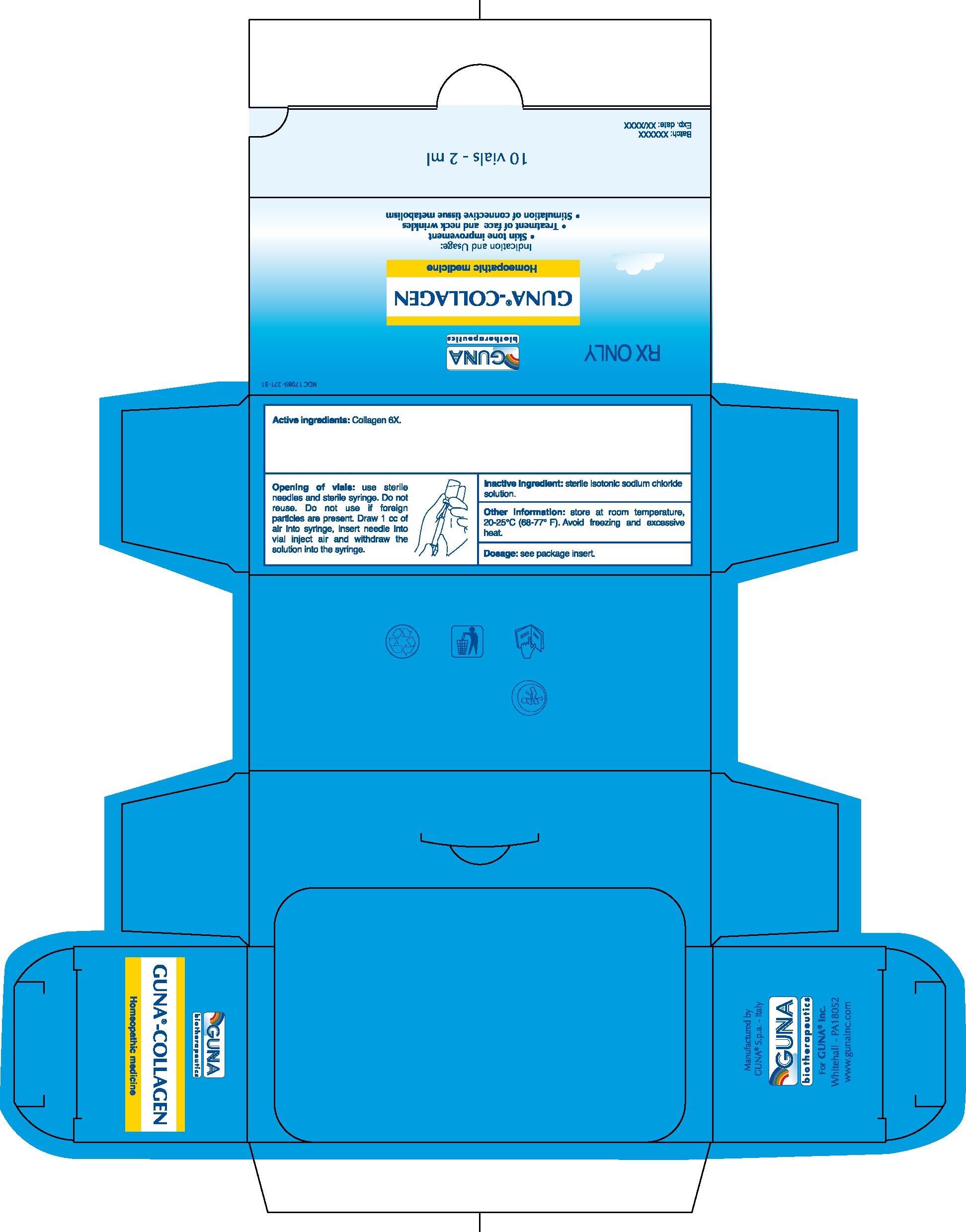
1. Indications and Usage for Guna Collagen
1.1. Skin tone improvement
1.2. Treatment of face and neck wrinkles
1.3. Stimulation of connective tissue metabolism.
2. Guna Collagen Dosage and Administration
2.1. Skin tone improvement for IM administration: 1 vial 1-3 times a week according to severity and clinical progress.
2.2. Treatment of face and neck wrinkles using mesotherapy technique: 1 vial. Using a 4 mm, 30G needle, make the classic intradermal injections utilizing mesotherapy technique.
2.3. Treatment of neck wrinkles using mesotherapy technique: 0.3 ml per wrinkle/cannulation. Using a 13 mm, 30G needle, insert the needle beneath the skin the full length of the needle, cannulate the wrinkle by moving the needle gently to left and right, while injecting the content of the syringe (tunnelling technique) as the needle is withdrawn.
2.4. Standard protocol according to mesotherapy technique consists of one session a week for 5-7 weeks. For prolonged treatments, 2 sessions for the first week, 1 session a week for 1 month and then 1 session a month are recommended.
Discard unused solution.
2.5. Opening of Vials: Use sterile needles and sterile syringe. Do not reuse. Do not use if foreign particles are present. Draw 1 cc of air into syringe, insert needle into vial inject air and withdraw the solution into the syringe.
3. Dosage Forms and Strengths
3.1. Injectable solution for subcutaneneous, intradermal, or intramuscular administration.
3.2. 2 ml glass vials
3.3. The active ingredient is manufactured according to the procedures stated in the European Pharmacopeia and the Official German Homeopathic Pharmacopeia.
Active ingredient: Collagen 6X.
Inactive ingredient: Sterile isotonic sodium chloride solution.
4. Contraindications
4.1. There is no history of hypersensitivity to GUNA®-COLLAGEN. However patients with a known hypersensitivity to any ingredient should be tested before use. Make a spot injection (0.1ml) into the forearm and observe for any reactions for 1 hour.
5. Warnings and Precautions
5.1. Be sure to disinfect the area before application. After treatment, avoid alcohol containing disinfectants as they produce local irritation that appears unpleasant on face. In most cases, the slight irritation disappears within 2 hours.
5.2. Skin disinfection is required before application. Saprophytic bacteria may produce injection site abscesses with improper skin preparation.
6. Adverse Reactions/Side Effects
6.1. The most common mild adverse reaction is slight reddening at the injection site due to the mechanical effect of the needle or a superficial skin reaction of mild erythema.
8. Use In Specific Populations
8.1 Pregnancy: Pregnancy category C. Animal reproduction studies have not been conducted with GUNA®-COLLAGEN. GUNA®- COLLAGEN should not be administered to a pregnant woman.
8.2 Nursing mothers: It is not known whether any of the ingredients in GUNA®- COLLAGEN are secreted in human milk. However, since many drugs are secreted in human milk, caution should be exercised when GUNA®- COLLAGEN is administered to a nursing woman.
8.3 Pediatric use: Effectiveness in paediatric patients has not been established
8.4 Geriatric use: No restrictions.
11. Guna Collagen Description
11.1. GUNA®-COLLAGEN is a sterile solution made with isotonic sodium chloride solution. The strength at 6X supports the physiological functions of collagen, as the homeopathic biological substrate acts as supporter of the endogenous production of collagen. Application locally utilizing mesotherapy technique targets the area needing physiologic support. GUNA®-COLLAGEN shows a “flattening” effect on wrinkles, this improves as the protocol is continued; the effect is due to improved metabolism in the area of application not due to a filler effect.
12. Guna Collagen - Clinical Pharmacology
12.1. Mechanism of Action
The medication acts through a low-dose enzymatic mechanism. Due to the homeopathic nature of the active ingredients, receptors are not blocked due to saturation of their active sites, rather there is a feed back regulation of the physiological metabolism of collagen.
12.2. Pharmacodynamics
The physiological effects of GUNA®-COLLAGEN are based on the activity of attenuated collagen that stimulates the metabolism of endogenous collagen.
In homeopathy there is no direct relationship between dose and effect, rather there is a relationship between attenuation and balancing effect on biochemical pathways.
12.3. Pharmacokinetics
The homeopathic attenuation provides complete bioavability of the active ingredients.
13. Nonclinical Toxicology
13.1. GUNA®-COLLAGEN has no level of toxicity due to the attenuation of the ingredients.
14. Clinical Studies
14.1. Collagen as an injectable application is widely used for treatment of wrinkles. The use of the homeopathic attenuation is an extension of the traditional application, where the effect on wrinkles is due to improvement of the metabolism of the area of application not to a filling activity. The skin gets a shining and vitalizing aspect that lasts from 3 to 6 months after the end of the protocol, depending on the patient’s habits and skin situation before treatment.
15. References
15.1. E.Italia, M. De Bellis: Manuale di Omeo-mesoterapia – Guna Ed. 1995
15.2. H.H. Reckeweg: Homeopathic Materia Medica . Aurelia Verlag
16. Storage and Handling
16.1. NDC 17089-271-31 10 glass vials packed in carton box16.2. NDC 17089-271-32 50 glass vials packed in carton box
16.3. Store at room temperature, 20-25°C (68-77° F). Avoid freezing and excessive heat.
| GUNA-COLLAGEN
sus scrofa collagen injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Guna spa (430538264) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Guna spa | 430538264 | manufacture | |